
For Healthcare ProfessionalsĪpplies to propofol: intravenous emulsion.
#ENDOSCOPY AFTER EFFECTS FULL#
For additional information, please see the full guidance at /regulatory-information/search-fda-guidance-documents/temporary-policy-repackaging-or-combining-propofol-drug-products-during-covid-19-public-health.Ĭommon adverse reactions (>1%): bradycardia, arrhythmia, tachycardia, hypotension, hypertension, decreased cardiac output, movement, apnea, respiratory acidosis during weaning, rash, pruritus, burning/stinging or pain at injection site, hyperlipemia. As relevant needs and circumstances evolve, FDA intends to update, modify, or withdraw policies in this guidance as appropriate. FDA is continually assessing the needs and circumstances that make issuance of this guidance appropriate. This policy is intended to remain in effect for no longer than the duration of the public health emergency related to COVID-19 declared by HHS, including any renewals made by the HHS Secretary in accordance with section 319(a)(2) of the Public Health Service Act (42 U.S.C. 353b) as outlined in this guidance for the duration of the public health emergency declared by the Secretary of Health and Human Services (HHS) on January 31, 2020, or for such shorter time as FDA may announce through updated guidance. FDA is issuing this guidance to communicate its temporary policy regarding the repackaging or combining of propofol drug products by a licensed pharmacist in a State licensed pharmacy, a Federal facility, or an outsourcing facility registered pursuant to section 503B of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. Temporary Policy on Repackaging or Combining Propofol Drug Products During the COVID-19 Public Health Emergency: 756 During the COVID-19 public health emergency, FDA has received several inquiries from healthcare professionals concerning the unavailability of propofol drug products used in the treatment and management of patients with complications related to COVID-19. For additional information, consult the EUA at and the fact sheet for healthcare providers at. The product should be used only in accordance with the conditions described in the authorization and with dosage regimens recommended in the fact sheet for healthcare providers. All rights reserved.Emergency Use Authorization (EUA) for Propofol-Lipuro: 759 760 On March 12, 2021, FDA issued an Emergency Use Authorization (EUA) permitting the emergency use of the unapproved product, Propofol-Lipuro 1% injectable emulsion for infusion, to maintain sedation via continuous infusion in patients older than 16 years of age who require mechanical ventilation in an intensive care unit (ICU) setting during the COVID-19 pandemic. Nasal bleeding Risk factors Transnasal endoscopy.Ĭopyright © 2017 Kaohsiung Medical University. Female and young patients are significantly associated with an increased risk of bleeding from transnasal endoscopy, but antiplatelet and/or anticoagulant medications and a history of chronic/allergic rhinitis may not be associated.

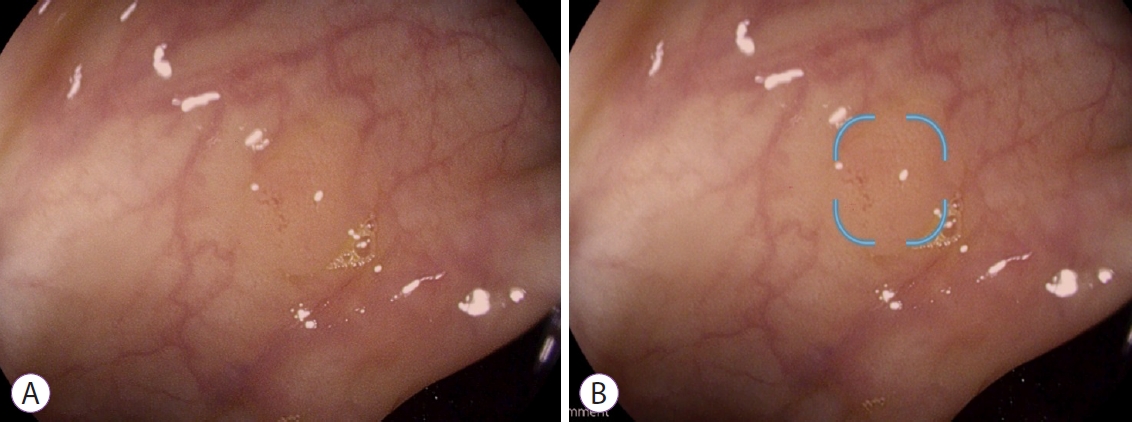
Other factors including the use of antiplatelet and/or anticoagulant drugs were not significantly associated with nasal bleeding. Multiple logistic regression revealed that nasal bleeding was significantly associated with age in decades, female gender (2.15, 95% CI 1.48-3.12, p < 0.001), a history of previous upper gastrointestinal endoscopy (0.55, 95% CI 0.36-0.82, p = 0.004), and chronic/allergic rhinitis (0.60, 95% CI 0.36-0.98, p = 0.043). Patient data were retrospectively evaluated including anthropometric, medical, and life-style parameters with multiple logistic regression analysis.
Nasal bleeding occurred in 160/3035 (5.3%) of patients undergoing transnasal endoscopy as part of health checkups.

The aim of this study is to identify risk factors for nasal bleeding during transnasal endoscopy. Nasal bleeding is the most severe adverse effect, but specific risk factors have not been identified. Transnasal endoscopy is widely used in screening for upper gastrointestinal lesions because of less associated pain.


 0 kommentar(er)
0 kommentar(er)
